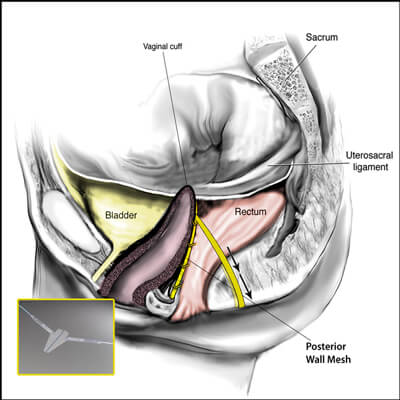
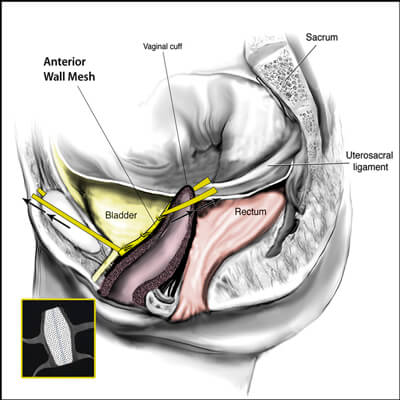
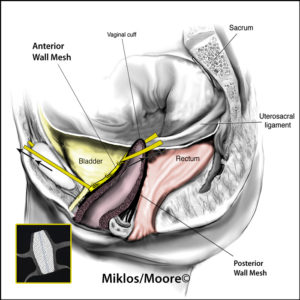
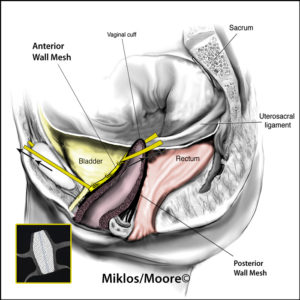
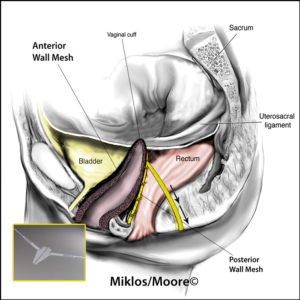
The transvaginal mesh surgical approaches were made popular because they were a simpler, faster and in theory a less complicated surgery when compared to the traditional abdominal sacrocolpopexy approach. Specifically, the anterior vaginal wall mesh is used to treat a cystocele or a bladder drop and a posterior vaginal wall mesh is used to treat the rectocele or a weakness in the floor of the vagina.
Additionally, these kits were developed by industry who then trained many surgeons to do the procedures which resulted in many inexperienced surgeons doing them and complications ensued. This led to an FDA notification in the fall of 2008 that notified patients to be aware of the complications that do potentially exist and to ensure your surgeon has the proper training and experience to do the procedures. A second 2011 FDA warning suggests that the transvaginal mesh should only be used after discussing the other options including: 1) do nothing 2) use of a pessary 3) native tissue reconstruction and 4) mesh used via an abdominal approach ie sacrocolpopexy.
Mesh has been proven at times to creates complications in a woman after its placement. It also is important to note that NO surgery is without risk of complications whether mesh is used or not. For more information about mesh complications and removal see 3 of the largest scientific papers in the world on mesh removal. Read our Google reviews.
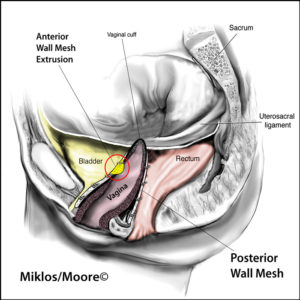
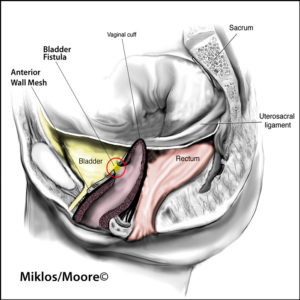
Transvaginal mesh implant can lead to vaginal mesh complications which are rare but can be devastating and life altering. These implant complications include and are not limited to: vaginal mesh extrusion, organ erosion, painful sex or vaginal pain.